Cell Therapy
(CAR-NK)
Next-generation CAR-NK therapy engineered for malignant gliomas
NK-optimized CAR design delivering potent tumor killing, validated in spheroid studies
Why CAR-NK cell?
CAR-NK therapy combines the innate, rapid tumor-killing power of natural killer (NK) cells with the precision of chimeric antigen receptors (CARs) — a promising, potentially safer “off-the-shelf” approach to target malignant glioma (high-grade/GBM)
Why we focus on CAR-NK for malignant glioma?
High unmet need: Malignant gliomas progress rapidly, recur frequently, and affect both adults and children.
Targetable and potent: Many gliomas express antigens (e.g., GD2); CAR-NKs combine antigen precision with NK cells’ rapid tumor-killing—even against MHC-low tumors.
Accessible and safer: Off-the-shelf CAR-NKs shorten time-to-treatment and carry lower risks of severe CRS/GVHD; reimbursement programs (e.g., V147) can significantly improve patient access.
Research Status
Development of CAR for NK Cell
- GD2: a tumor-specific antigen widely expressed in neural-derived cancers
- Clinically validated target of the FDA-approved drug Dinutuximab
- NK-optimized GD2-CAR enables strong anti-tumor activity in difficult CNS tumors such as astrocytoma
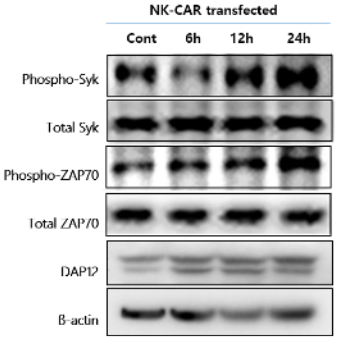
Verification of CAR-NK Activation (SYK Phosphorylation)
- Tumor antigen recognition triggers ITAM signaling
- ITAM phosphorylation allows SYK binding and activation
- Activated SYK initiates downstream PLCγ, MAPK, and PI3K-AKT pathways
- Leads to enhanced cytotoxic activity and cytokine secretion
- SYK phosphorylation serves as molecular evidence of CAR-NK activation
Western blot shows increased phosphorylation of SYK and ZAP70 after CAR-NK activation, confirming engagement of NK signaling pathways